06 Feb Behind an innovative new blood testing technology that, unlike Theranos, is real
The Theranos debacle cast a shadow over emerging blood testing technologies once it became clear the startup’s marquee platform device, which claimed the ability to conduct complex blood analysis with just a few drops of patients’ blood, was a sham and led to the eventual imprisonment of founder Elizabeth Homes in an 11-year jail sentence. But Becton Dickinson and Co. (BD), a long-standing global diagnostics and medical supply giant whose 75-year pedigree includes developing at-home COVID testing kits at the height of the pandemic, is now claiming the mantle for the next generation of blood tests. They can be conducted in local settings like pharmacies with just pinpricks of blood–and the company has the FDA regulatory clearances and initial pharmacy partnerships to prove it’s not just smoke and mirrors, the company told Fast Company in an exclusive interview.
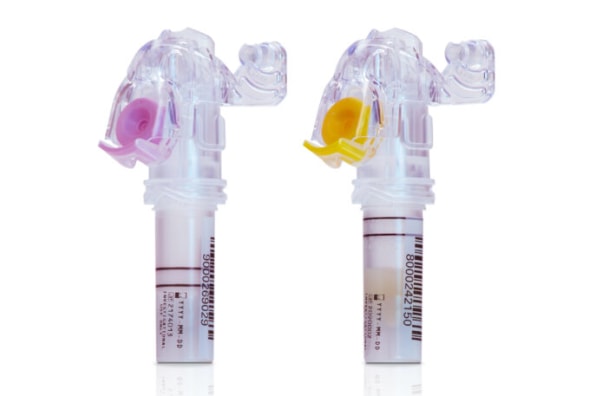
BD received its FDA authorizations for two blood draw devices that are part of the new BD MiniDraw Capillary Blood Collection System at the end of 2023. The devices, as the name implies, collect blood on a capillary level rather than through larger traditional vials which draw blood from your veins–and have the ability to conduct a number of chemical and molecular tests with just 16 to 18 drops, according to Dave Hickey, executive vice resident and president of BD’s life sciences segment.
While that tech is itself noteworthy for its convenience, its broader ramifications lay in the democratizing access to the critical medical information that resides in patients’ veins. For instance, it could allow a pharmacist to conduct regular blood draws locally, rather than having to see a specially-trained nurse or professional phlebotomist at a lab or hospital.
“I’ve literally had a bit of the BD MiniDraw experience,” he tells Fast Company. “It was simple, it was convenient. For me, it was virtually pain free–it uses a BD blood draw lancet integrated into the device. But what sticks out is the fact that I was in a decentralized location, and they take just literally 15 to 16 drops of blood, within a couple of minutes, to do the tests.”
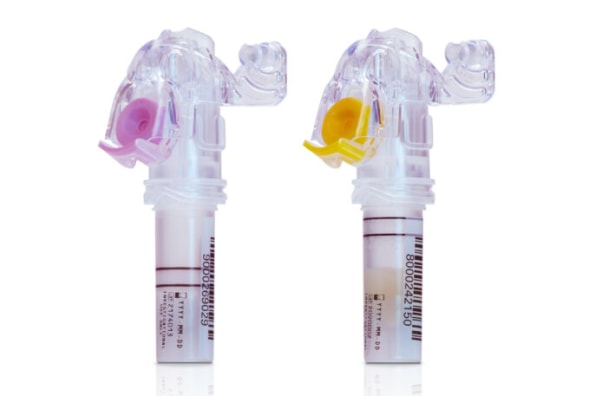
For now, the MiniDraw devices can conduct common and relatively simple tests that use “low volume blood collection for lipid panels, selective chemistry tests, hemoglobin and hematocrit tests,” according to Hickey. “We prioritize those, because of course, those tests are the most commonly ordered ones.” Further testing capacity could follow as the technology is further developed by BD and its partners. For instance, more complex molecular tests that rely on anti-coagulation techniques (which one of the two FDA-cleared MiniDraw devices is specifically built for) could theoretically help in conducting non-invasive screenings for diseases like cancer, though these are currently not in development and BD says there’s no definite timeline for such milestones.
But BD’s broader vision both in the U.S. and beyond is tied to the democratization of blood testing and what it might mean for public health. In America, for instance, the COVID pandemic and concurrent rise of telehealth has shifted the dynamics of diagnostics. Patients may not want to hustle to an outpatient facility or lab, or wait for days or weeks, to get basic updates on the blood chemistry numbers critical to monitoring both chronic conditions and more immediate medical threats. A trek to the local pharmacist trained with a simple video could be a valuable proposition.
“Up to 70% of clinical decisions are based on information derived from lab test results,” says Hickey. “Even today, we have people that are not getting access to this market that is worth over $2 billion annually.”
Connecting with other diagnostics specialists who can further develop and deploy the MiniDraw tech is also critical for such an ecosystem. BD has an existing partnership with Austin, Texas-based Babson Diagnostics for its initial MiniDraw efforts. Under the partnership, Babson’s own blood diagnostic technologies will be used to prepare samples, and the companies plan to roll out the services to pharmacies later this year.
Globally, the technology presents additional opportunities, as Dickey points out. In developing nations with little healthcare infrastructure, the ability to conduct, say, an HPV screening blood test in the privacy of one’s own home rather than face certain societal taboos could prove a game-changer in its own right.
“When you look at the trend towards these rapidly changing patient expectations—when you think of telehealth and COVID, testing at home, and at-home diagnostic testing—we don’t see blood collection as any different,” says Dickey. “I think patients are going to want to continue to have a better experience more access, and eventually, even in their homes.”
Source: Fast Company


Sorry, the comment form is closed at this time.